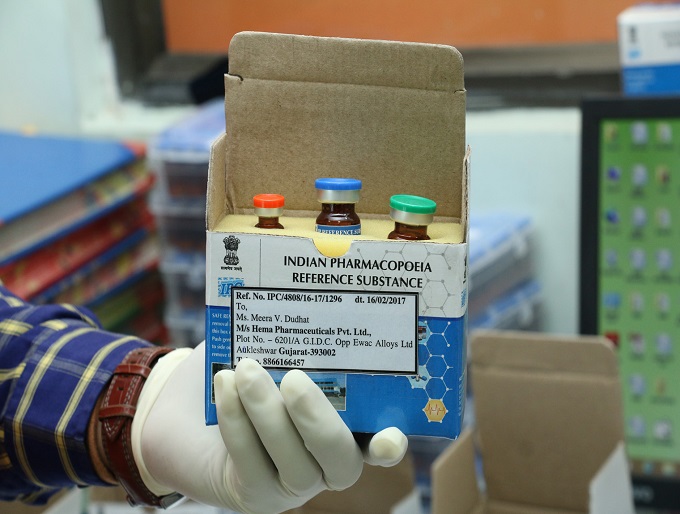
welcome to IPC Labs
Indian Pharmacopoeia Laboratory is accredited for both chemical and biological testing from Sept.2011 by National Accreditation Board for testing and calibration laboratories (NABL) of DST, New Delhi for complying ISO/IEC 17025: 2005.
IPL is well equipped with modern analytical instruments like HPLC, HPTLC, GC, GC-MS, GCHS, FTIR, TGA-DSC, LC-MSMS, ICP-MS, CHN-S, NMR, Polarimeter, Autotitrator, KF Titrator,Microbalances etc.
read more
IPC Lab Sample Testing Workflow
Sampling/Reciept/Storage
Manage sampling points, receive samples, allocate storage location & manage custody.
Specification Management
Specifications creation,approval and managment,MDL,Formula.
Sample Registration
Sample registration,allocaion,approval and barcoding of the samples.
Result(Manual/ELN)
Results published are subject to release with two levels of review and approval.
Approval
Configurable approval workflows for different sample types.
Traceability
Built-in audit trail, access to instrument data, inventory usage, comments, re-tests.
Sample Tested Till Date at IPC Labs
1562
Drugs
609
Vaccine
4156
Medical Devices
1500
Cosmetics